tupungato/iStock through Getty Photographs
Mid- and late-stage knowledge readouts over the previous couple of years for Alzheimer’s therapies have had an analogous theme: Failure.
Every one of these candidates have one factor in frequent: They aim decreasing beta-amyloid plaque within the mind that’s thought to play a task within the memory-robbing illness.
Nonetheless, the amyloid-targeting theme is dropping steam as evidenced by mounting failures. The latest was in June when Roche (OTCQX:RHHBY) reported that crenezumab didn’t gradual or stop cognitive decline in individuals with a genetic mutation that causes early-onset Alzheimer’s.
Roche (OTCQX:RHHBF) can also be anticipated to launch section 3 knowledge on one other candidate, gantenerumab in early Alzheimer’s, in This fall. Gantenerumab, like crenezumab, each goal amyloid.
The crenezumab failure got here after the flop of Biogen’s Aduhelm (aducanumab). Though it received approval in 2021, in April, the Facilities for Medicare & Medicaid Providers severely restricted protection of the therapy citing poor efficacy. It too is an amyloid-targeting remedy.
Eli Lilly (NYSE:LLY) is creating donanemab although the drugmaker will not have section 3 knowledge out there till mid-2023. Nonetheless, section 2 knowledge confirmed statistically vital slowing of scientific development and a discount in amyloid plaque.
Lilly’s (LLY) different Alzheimer’s candidate, solanezumab, failed in three section 3 trials. The monocolonal antibody works by binding to beta-amyloid peptides after which flushing plaque out of the mind.
Biogen (NASDAQ:BIIB) and associate Eisai (OTCPK:ESALY) are additionally pinning hopes that their amyloid-plaque focusing on monoclonal antibody lecanemab will fare higher than aducanumab. They accomplished a rolling submission to the FDA in Could.
Nonetheless, many different corporations are centered on candidates that are not focusing on beta-amyloid plaque.
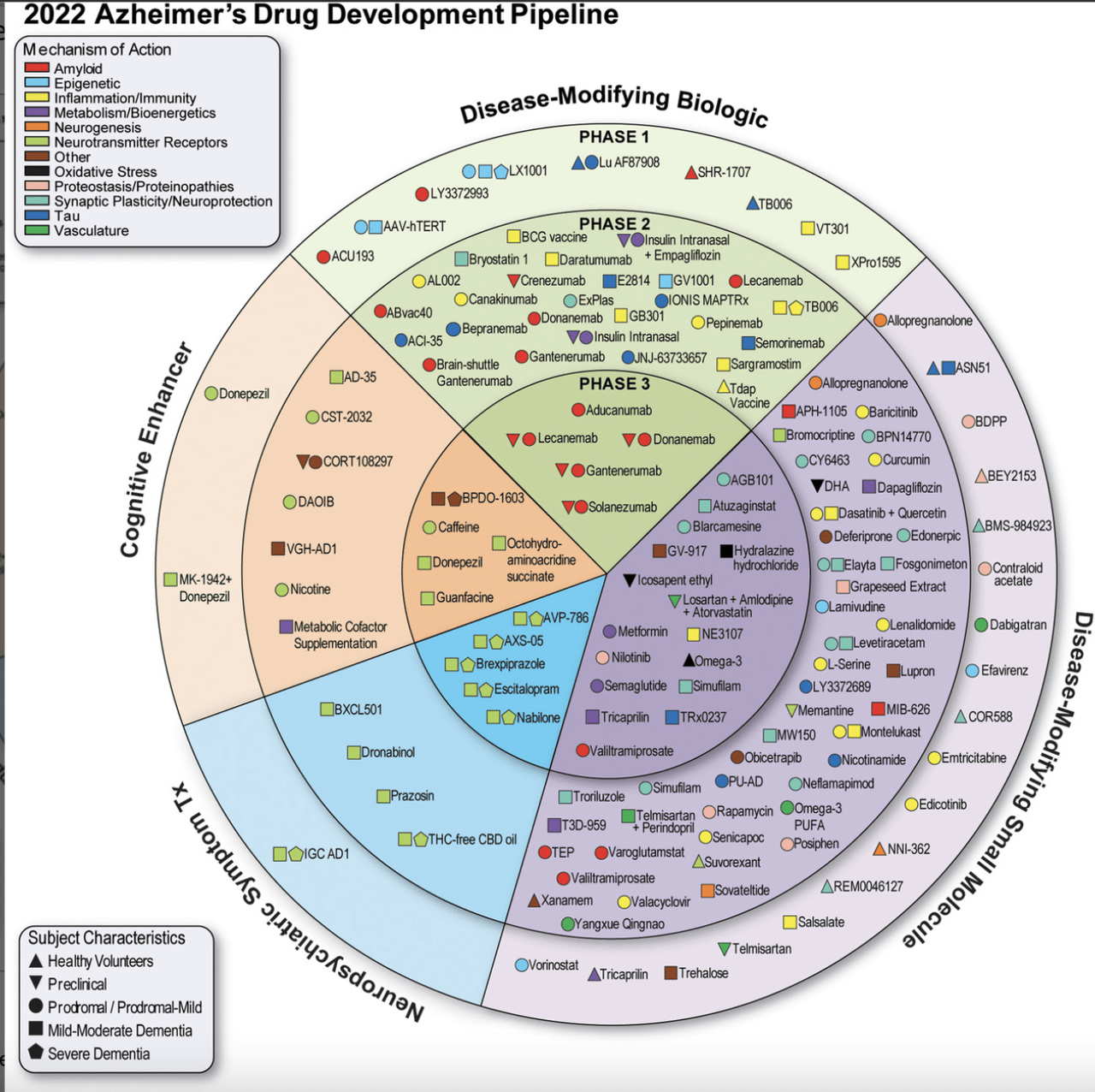
By way of late-stage candidates, some of the promising — and controversial — that matches this invoice is simufilam from Cassava Sciences (NASDAQ:SAVA). In contrast to most different Alzheimer’s therapies in growth, simufilam is given orally. The corporate says the drug works by restoring the traditional form and performance of altered filamin A, a scaffolding protein, within the mind.
Nonetheless, knowledge from a number of research has come beneath fireplace and a scientific journal retracted 5 studied about simufilam authored by a Cassava (SAVA) advisor. Lane Simonian, an Alzheimer’s researcher and Searching for Alpha contributor, famous that traders ought to maintain off on shopping for shares in Cassava (SAVA) and there are possible higher remedies for Alzheimer’s.
Anavex Life Sciences Corp. (AVXL) has ANAVEX2-73 (blarcamersine), an oral answer that can also be in late-stage growth. The drug prompts the Sigma-1 receptor, which is thought to play a task in regulating neurodegeneration. By doing so, it could reduce protein misfolding and ease oxidative stress in mind cells.
High-line knowledge from a section 2b/3 research is anticipated in H2 2022.
Although Anavex (AVXL) was down ~43% in H1, Lane Simonian argues that blarcamesine’s mechanism of motion distinguishes it from different candidates and holds promise.
Alector (ALEC) has ALN002 which targets TREM2, a gene that provides directions for making the protein triggering receptor discovered on myeloid cells. ALN002 works by bettering TREM2 signaling, enhancing microglia exercise.
Alector (ALEC) is partnered with AbbVie (ABBV) on the section 2 candidate.
The corporate can also be creating AL001 to deal with genetically pushed progranulin deficiencies that are considered a trigger for frontotemporal dementia and a danger issue for situations equivalent to Alzheimer’s. It’s partnered with GlaxoSmithKline (GSK) on this candidate.
In March, Stifel reduce Alector (ALEC) to carry from purchase, expressing warning on AL001.
Buntanetap (previously ANVS401), yet one more oral drug for Alzheimer’s, is in section 2 growth from Annovis Bio (ANVS). It really works by inhibiting neurotoxic proteins that kill nerve cells. In human and animal research, it has demonstrated the flexibility to decrease ranges of amyloid beta precursor protein and amyloid beta, tau/phospho-tau, and α-synuclein.
In section 2a trials, the candidate was discovered to be protected and achieved acceptable pharmacokinetic ranges, assembly each main endpoints. As well as, enchancment in cognition was additionally seen.
AC Immune (ACIU) has two Alzheimer’s candidates within the clinic: The anti-tau antibody semorinemab in section 2 and the anti-pTau vaccine ACI-35.030. The previous is partnered with Roche’s (OTCQX:RHHBY) Genentech unit, whereas the latter is with Johnson & Johnson’s (JNJ) Janssen division.
Semorinemab was dealt a setback in November 2021 after it missed considered one of its co-primary endpoints in a section 2 trial. Nonetheless, AC Immune (ACIU) noticed optimistic interim outcomes for ACI-35.030 in a Part 1b/2a trial involving aged sufferers with early Alzheimer’s illness in February 2021.
Acumen Prescription drugs (ABOS) is value being attentive to for ACU193. Though simply in section 1, the candidate works in another way from different Alzheimer’s therapies as an anti-amyloid-beta oligomer (AβO) monoclonal antibody. The corporate touts that in consequence, it has a greater security profile, in addition to the potential for cognitive enchancment and illness slowing.
H.C. Wainwright just lately initiated the inventory with a purchase score, projecting greater than 200% upside.
Athira’s Pharma’s (ATHA) fosgonimeton (ATH-1017) is designed increase the exercise of hepatocyte progress issue and its receptor, MET which can be expressed within the central nervous system. That is thought to advertise mind well being and performance.
In June, nonetheless, the corporate reported {that a} mid-stage trial of the candidate in sufferers with mild-to-moderate Alzheimer’s failed each main and secondary endpoints.