After we first encountered the thrilling world of artificial biology, we couldn’t wait to put money into the chief on the time – Intrexon. Then, when the corporate’s fearless chief spent an earnings name speaking about how a lot cash they deliberate to make promoting apple slices, we exited our place and realized that the majority artificial biology shares had been failing miserably on the execution aspect. Immediately, we’re approaching the standard synbio suspects – Zymergen, Amyris, and Ginkgo Bioworks – with a substantial amount of trepidation. Whereas we see indicators of promise for Ginkgo’s enterprise mannequin, we’re sitting on the sidelines for now.
As we wait to see how Ginkgo progresses, we determined to vet our present number of gene enhancing shares to see what we personal and why. Admittedly, the “why” was merely a spray-and-pray method the place we invested within the first three CRISPR shares to go public – Intellia (NTLA), Crispr Therapeutics (CRSP), and Editas Medication (EDIT) – and left it at that. Then, we revisited these three corporations final 12 months in a chunk titled What Do The Three Greatest Gene Enhancing Shares Do? which concluded that Editas wasn’t seeing the identical success as the opposite two corporations with their two flagship therapies.
We rely closely on the success of EDIT-101 and EDIT-301. Apart from EDIT-101 and EDIT-301, all of our product growth packages are on the preclinical or analysis stage.
Editas Medication 10-Ok
This makes issues simple. If both of those therapies fail, the probability traders will take a punt on extra merchandise appears extremely unlikely. It’s all or nothing for EDIT-101 and EDIT-301 that are known as the BRILLIANCE and RUBY trials respectively.
Brilliance: EDIT-101
The one human dosing that’s taken place to date is for EDIT-101 which started in 2020. Says the corporate:
EDIT-101 for the therapy of LCA10 has a restricted affected person pool from which to attract for enrollment in a medical trial, as the worldwide incidence of LCA10 is estimated to be two to a few per 100,000 reside births worldwide.
Editas Medication
The supply of sufferers is even decrease than what’s being said above. Leber Congenital Amaurosis, or LCA, is a gaggle of inherited retinal degenerative issues brought on by mutations in no less than 18 totally different genes. The most typical type of the illness, LCA10, is a monogenic dysfunction brought on by mutations within the CEP290 gene and is the reason for illness in roughly 20‑30% of all LCA sufferers. The NIH estimates there to be 3,000 to 30,000 individuals with the illness, so the full addressable marketplace for EDIT-101 is 10,000 sufferers utilizing essentially the most optimistic estimates (30% X 30,000).
After two years of labor, the primary outcomes of the BRILLIANCE examine had been made out there for the small pattern of six sufferers who participated (2 low dose, 4 mid dose) and so they’re surprisingly accessible to the typical lay individual, although not overly spectacular in response to pundits.
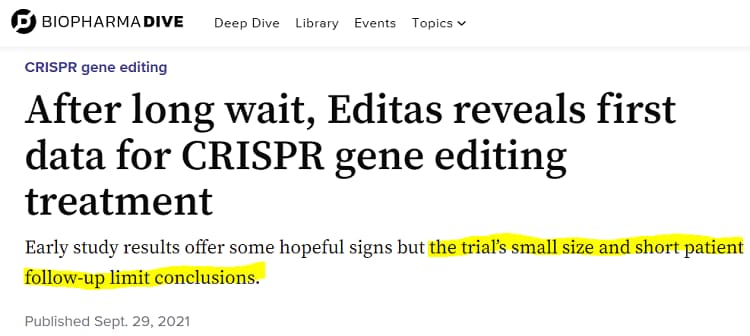
Since so few sufferers are enrolled, you may really learn the outcomes for every in regard to security (did it hurt the sufferers?) and efficacy (did it assist the sufferers). Spoiler alert: it’s secure however efficacy is exhibiting combined outcomes. The trial is being performed utilizing escalating doses (low, mid, excessive), and a few sufferers from the center dosage cohort appear to be realizing noticeable advantages. The larger query on everybody’s thoughts is why Editas Medication’s pharmaceutical companion for EDIT-101, Allergan, backed out a month prior to those outcomes being launched. The foundation trigger was a call made by AbbVie (ABBV), the $257 billion pharma firm that acquired Allergan (AbbVie has since sidled as much as Caribou). In response to this setback, Editas tried to show lemons into lemonade.
“We’re happy to regain full working management of our ocular packages, together with EDIT-101, the primary in vivo CRISPR drugs to be administered to sufferers, and we look ahead to creating and commercializing these transformative ocular medicines.
Cynthia Collins, CEO of Editas Medication on the time
Ah, the previous “our pharma companion ditched us and we’re actually stoked to go at it alone” assertion. Ms. Collins is only one of many executives that Editas has seen come and go over time, one other crimson flag that may’t be ignored.
The Government Turnover Downside
Inside turmoil signifies an organization that’s extra centered on infighting than precise outcomes. Perhaps they need to take into account placing their D&I objectives apart and hiring primarily based purely on competency? Then once more, which competent individual would have a look at this firestorm of turnover and assume it’s an incredible surroundings for profession development? Executives coming and going can’t be serving to stabilize any of the remaining pharma partnerships both.
- March 2019 – CEO Katrine Bosley departs following beforehand introduced exits of the corporate’s chief medical and chief monetary officers. Since then, nearly all of Editas’ government staff has been changed. Bosley was appointed CEO in mid-2014, roughly half a 12 months after the corporate was based.
- Feb 2021 – CEO Cynthia Collins departs after lasting 1.5 years handing over the reins to James Mullen
- Feb 2022 – CMO Lisa Michaels fired after 15 months on the job. Editas has had three totally different chief medical officers since late 2016.
- June 2022 – CEO Gilmore O’Neil takes over the reins from James Mullen and is claimed to be on the lookout for new CMO
In the event you’re a big pharma firm on the lookout for a gene-editing companion, you’d need to undergo some critical psychological gymnastics to miss all of the soiled laundry that’s been aired as Editas modifications C-level positions extra usually than Kim Kardashian modifications outfits.
RUBY: EDIT-301
Simply final month, the FDA granted Orphan Drug Designation to EDIT-301 for the therapy of beta thalassemia and sickle cell illness. Take a look at this glorious visible depiction which helps clarify illnesses that consequence from mutations in a gene that encodes a key part of hemoglobin, the oxygen-carrying molecule in blood.
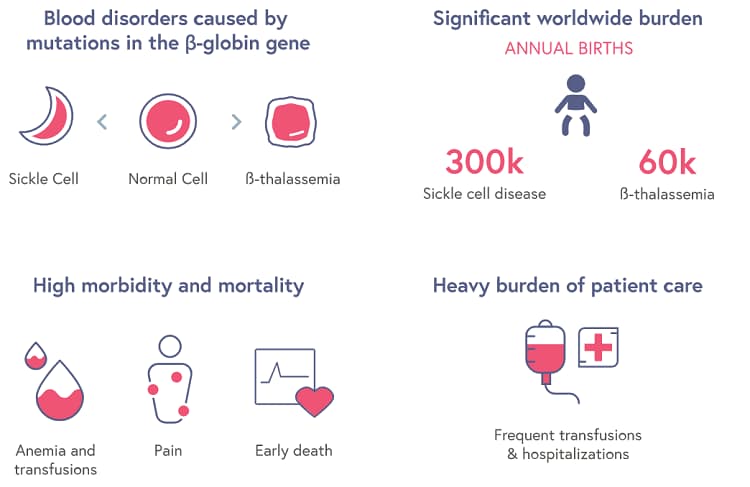
The corporate that produced the above graphic, CRISPR Therapeutics, has their very own gene-editing therapy for sufferers with both beta thalassemia or sickle cell illness which is at the moment being investigated in a number of ongoing medical trials as a possible one-time remedy for sufferers. The beneath excerpt from a November 2019 BioPharma Dive article exhibits simply how far forward of Editas CRISPR Therapeutics is:
9 months after receiving an infusion of gene-edited stem cells, a affected person in a intently adopted medical examine is free from the blood transfusions mandatory for individuals who reside with extreme beta-thalassemia, an inherited illness brought on by faulty crimson blood cells.
Credit score: BioPharma Dive, First have a look at CRISPR, Vertex gene-editing remedy hints at therapy potential
Three years later, and Editas is on monitor to dose their first transfusion-dependent beta thalassemia affected person with EDIT-301 by the tip of 2022. Since we’re already holding shares of CRISPR Therapeutics, publicity to EDIT-301 appears pointless.
Following the Founders
What attracted us to Editas within the first place was the high-profile founders who’re thought-about to be pioneers within the gene-editing house – David Liu, J. Keith Joung, Feng Zhang, and George Church. Except for George Church, all of those founders went on to discovered one other firm known as Beam Therapeutics (BEAM) which wields a gene-editing know-how known as “base enhancing” that’s stated to be superior to all different strategies. Whereas George Church wasn’t concerned on this discovery, right here’s what he needed to say about it when the invention was revealed again in 2016:
Due to “the cell’s determined makes an attempt” to fix its genome, stated Harvard College biologist George Church, “what usually passes as ‘genome enhancing’ would extra appropriately be known as ‘genome vandalism,’” because the cell inserts and deletes random bits of DNA the place CRISPR cuts it. As a result of the brand new model of CRISPR avoids that mess, it “provides an enormous step ahead,” stated Church, who was not concerned within the discovery, and whose 2013 paper helped launch the CRISPR frenzy. “It’s arguably essentially the most intelligent CRISPR gadget so far.”
Credit score: STAT, Scientists unveil the ‘most intelligent CRISPR gadget’ to date
A 12 months later, Beam Therapeutics was based, however that’s a narrative we’ll save for one more day.
Conclusion
After compiling A Full Listing of 27 Gene Enhancing Shares, we lowered the listing to 5 shares that present pure-play methods for traders to get publicity to gene enhancing. Editas was a kind of names, however at the moment’s findings imply we’ll be altering it from a “love” to an “keep away from” as we exit our place and transfer to put money into a gene-editing firm that exhibits larger promise. In future articles, we’ll have a look at the promise of the remaining 4 gene-editing shares we shortlisted.
Tech investing is extraordinarily dangerous. Reduce your danger with our inventory analysis, funding instruments, and portfolios, and discover out which tech shares it is best to keep away from. Develop into a Nanalyze Premium member and discover out at the moment!